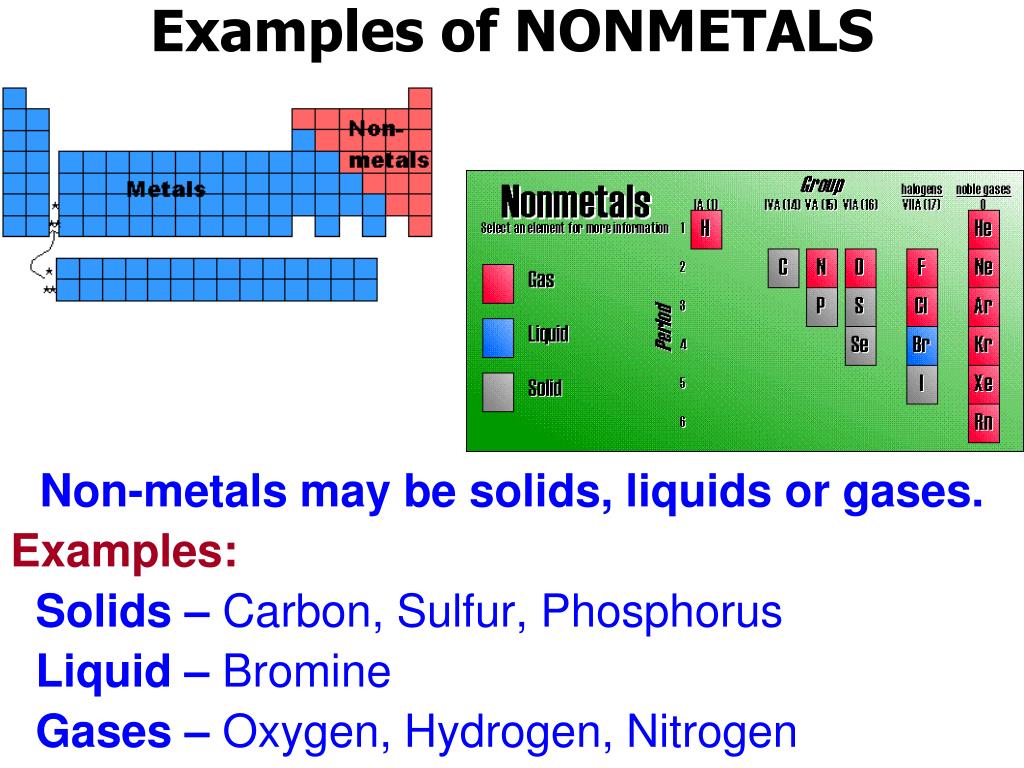
Are Metals Nonmetals Or Metalloids Ductile . Web most metals share the properties of being shiny, very dense, and having high melting points. Some metalloids, such as silicon and germanium, can act as. Metals are also good conductors of heat and electricity. Web they can form alloys with other metals. Metalloids have electronegativity values intermediate between metals and These have no metallic luster and do not reflect light Furthermore, they are ductile, malleable, and lustrous. Web like nonmetals, metalloids are neither malleable nor ductile. All metals are solids at Web metal and nonmetal elements have very different chemical and physical properties because they are made up of. They are poor conductors of heat and electricity. While solid at room temperature, metalloids have lower melting points than most metals.
from www.slideserve.com
All metals are solids at Web they can form alloys with other metals. Some metalloids, such as silicon and germanium, can act as. Web like nonmetals, metalloids are neither malleable nor ductile. They are poor conductors of heat and electricity. Metals are also good conductors of heat and electricity. These have no metallic luster and do not reflect light Furthermore, they are ductile, malleable, and lustrous. Metalloids have electronegativity values intermediate between metals and While solid at room temperature, metalloids have lower melting points than most metals.
PPT Metals, Nonmetals and Metalloids PowerPoint Presentation, free
Are Metals Nonmetals Or Metalloids Ductile Web they can form alloys with other metals. While solid at room temperature, metalloids have lower melting points than most metals. Web metal and nonmetal elements have very different chemical and physical properties because they are made up of. Metalloids have electronegativity values intermediate between metals and Web like nonmetals, metalloids are neither malleable nor ductile. Metals are also good conductors of heat and electricity. These have no metallic luster and do not reflect light Web they can form alloys with other metals. They are poor conductors of heat and electricity. Web most metals share the properties of being shiny, very dense, and having high melting points. All metals are solids at Some metalloids, such as silicon and germanium, can act as. Furthermore, they are ductile, malleable, and lustrous.
From slideplayer.com
Metals, Nonmetals and Metalloids ppt download Are Metals Nonmetals Or Metalloids Ductile Web metal and nonmetal elements have very different chemical and physical properties because they are made up of. These have no metallic luster and do not reflect light Metalloids have electronegativity values intermediate between metals and Web like nonmetals, metalloids are neither malleable nor ductile. They are poor conductors of heat and electricity. Web they can form alloys with other. Are Metals Nonmetals Or Metalloids Ductile.
From www.meadmetals.com
What’s the Difference Between Metals, Nonmetals, and Metalloids? Are Metals Nonmetals Or Metalloids Ductile Metals are also good conductors of heat and electricity. Some metalloids, such as silicon and germanium, can act as. These have no metallic luster and do not reflect light Web metal and nonmetal elements have very different chemical and physical properties because they are made up of. They are poor conductors of heat and electricity. All metals are solids at. Are Metals Nonmetals Or Metalloids Ductile.
From slideplayer.com
Metals, Nonmetals, and Metalloids ppt download Are Metals Nonmetals Or Metalloids Ductile They are poor conductors of heat and electricity. Web metal and nonmetal elements have very different chemical and physical properties because they are made up of. All metals are solids at Metalloids have electronegativity values intermediate between metals and While solid at room temperature, metalloids have lower melting points than most metals. Some metalloids, such as silicon and germanium, can. Are Metals Nonmetals Or Metalloids Ductile.
From www.youtube.com
Difference between Metals , Metalloids , and Nonmetals YouTube Are Metals Nonmetals Or Metalloids Ductile Web they can form alloys with other metals. Metalloids have electronegativity values intermediate between metals and While solid at room temperature, metalloids have lower melting points than most metals. Web most metals share the properties of being shiny, very dense, and having high melting points. All metals are solids at Some metalloids, such as silicon and germanium, can act as.. Are Metals Nonmetals Or Metalloids Ductile.
From www.teachoo.com
Metals, Non Metals and Metalloids Meaning & Difference Teachoo Are Metals Nonmetals Or Metalloids Ductile All metals are solids at Web they can form alloys with other metals. Metalloids have electronegativity values intermediate between metals and Web like nonmetals, metalloids are neither malleable nor ductile. They are poor conductors of heat and electricity. These have no metallic luster and do not reflect light Metals are also good conductors of heat and electricity. Some metalloids, such. Are Metals Nonmetals Or Metalloids Ductile.
From www.slideserve.com
PPT Metals, Nonmetals, Metalloids PowerPoint Presentation, free Are Metals Nonmetals Or Metalloids Ductile Web like nonmetals, metalloids are neither malleable nor ductile. Web most metals share the properties of being shiny, very dense, and having high melting points. While solid at room temperature, metalloids have lower melting points than most metals. These have no metallic luster and do not reflect light Web metal and nonmetal elements have very different chemical and physical properties. Are Metals Nonmetals Or Metalloids Ductile.
From www.youtube.com
How to identify METALS NONMETALS and METALLOIDS on the PERIODIC TABLE Are Metals Nonmetals Or Metalloids Ductile Web they can form alloys with other metals. Furthermore, they are ductile, malleable, and lustrous. Web most metals share the properties of being shiny, very dense, and having high melting points. Some metalloids, such as silicon and germanium, can act as. Metalloids have electronegativity values intermediate between metals and Metals are also good conductors of heat and electricity. These have. Are Metals Nonmetals Or Metalloids Ductile.
From www.slideserve.com
PPT Metals, Nonmetals, Metalloids PowerPoint Presentation, free Are Metals Nonmetals Or Metalloids Ductile Furthermore, they are ductile, malleable, and lustrous. Web they can form alloys with other metals. Web like nonmetals, metalloids are neither malleable nor ductile. They are poor conductors of heat and electricity. While solid at room temperature, metalloids have lower melting points than most metals. Web most metals share the properties of being shiny, very dense, and having high melting. Are Metals Nonmetals Or Metalloids Ductile.
From mungfali.com
Metals Nonmetals And Metalloids Chart Are Metals Nonmetals Or Metalloids Ductile Metals are also good conductors of heat and electricity. While solid at room temperature, metalloids have lower melting points than most metals. Web metal and nonmetal elements have very different chemical and physical properties because they are made up of. Web they can form alloys with other metals. These have no metallic luster and do not reflect light Web most. Are Metals Nonmetals Or Metalloids Ductile.
From www.slideserve.com
PPT Metals, Nonmetals, and Metalloids PowerPoint Presentation, free Are Metals Nonmetals Or Metalloids Ductile They are poor conductors of heat and electricity. Metalloids have electronegativity values intermediate between metals and Web like nonmetals, metalloids are neither malleable nor ductile. All metals are solids at Furthermore, they are ductile, malleable, and lustrous. While solid at room temperature, metalloids have lower melting points than most metals. Web metal and nonmetal elements have very different chemical and. Are Metals Nonmetals Or Metalloids Ductile.
From www.differencebetween.net
Difference Between Metals, Metalloids, and Nonmetals Difference Between Are Metals Nonmetals Or Metalloids Ductile All metals are solids at Web like nonmetals, metalloids are neither malleable nor ductile. Furthermore, they are ductile, malleable, and lustrous. While solid at room temperature, metalloids have lower melting points than most metals. Metals are also good conductors of heat and electricity. Some metalloids, such as silicon and germanium, can act as. Web metal and nonmetal elements have very. Are Metals Nonmetals Or Metalloids Ductile.
From utedzz.blogspot.com
Periodic Table With Metals Metalloids And Nonmetals Labeled Periodic Are Metals Nonmetals Or Metalloids Ductile These have no metallic luster and do not reflect light They are poor conductors of heat and electricity. All metals are solids at Web metal and nonmetal elements have very different chemical and physical properties because they are made up of. Web like nonmetals, metalloids are neither malleable nor ductile. Web they can form alloys with other metals. Metalloids have. Are Metals Nonmetals Or Metalloids Ductile.
From www.slideserve.com
PPT Metals, Nonmetals, and Metalloids PowerPoint Presentation, free Are Metals Nonmetals Or Metalloids Ductile Furthermore, they are ductile, malleable, and lustrous. Metals are also good conductors of heat and electricity. Web most metals share the properties of being shiny, very dense, and having high melting points. Web metal and nonmetal elements have very different chemical and physical properties because they are made up of. Web they can form alloys with other metals. These have. Are Metals Nonmetals Or Metalloids Ductile.
From pediaa.com
Difference Between Metals Nonmetals and Metalloids Definition Are Metals Nonmetals Or Metalloids Ductile While solid at room temperature, metalloids have lower melting points than most metals. Metals are also good conductors of heat and electricity. Web like nonmetals, metalloids are neither malleable nor ductile. Web they can form alloys with other metals. Metalloids have electronegativity values intermediate between metals and All metals are solids at These have no metallic luster and do not. Are Metals Nonmetals Or Metalloids Ductile.
From periodictableguide.com
Periodic table labeled with Metals Nonmetals and Metalloids Are Metals Nonmetals Or Metalloids Ductile Web most metals share the properties of being shiny, very dense, and having high melting points. Metals are also good conductors of heat and electricity. All metals are solids at Some metalloids, such as silicon and germanium, can act as. Web metal and nonmetal elements have very different chemical and physical properties because they are made up of. Furthermore, they. Are Metals Nonmetals Or Metalloids Ductile.
From www.slideserve.com
PPT Physical Properties Notes PowerPoint Presentation, free download Are Metals Nonmetals Or Metalloids Ductile While solid at room temperature, metalloids have lower melting points than most metals. Web metal and nonmetal elements have very different chemical and physical properties because they are made up of. All metals are solids at Web like nonmetals, metalloids are neither malleable nor ductile. Some metalloids, such as silicon and germanium, can act as. Metals are also good conductors. Are Metals Nonmetals Or Metalloids Ductile.
From www.dcicomp.com
Physical Properties Of Metals And Nonmetals And Metalloids Are Metals Nonmetals Or Metalloids Ductile These have no metallic luster and do not reflect light Metals are also good conductors of heat and electricity. All metals are solids at Some metalloids, such as silicon and germanium, can act as. Web most metals share the properties of being shiny, very dense, and having high melting points. Furthermore, they are ductile, malleable, and lustrous. Web they can. Are Metals Nonmetals Or Metalloids Ductile.
From slideplayer.com
Metals, Nonmetals, and Metalloids ppt download Are Metals Nonmetals Or Metalloids Ductile Web most metals share the properties of being shiny, very dense, and having high melting points. Web metal and nonmetal elements have very different chemical and physical properties because they are made up of. While solid at room temperature, metalloids have lower melting points than most metals. All metals are solids at These have no metallic luster and do not. Are Metals Nonmetals Or Metalloids Ductile.